The Mole and the Mass of Substances
- AinArifin
- Mar 17, 2017
- 1 min read
The mass of one mole of any substance is called molar mass
Units: g mol-1
The molar mass of substances are numerically equal to relative mass
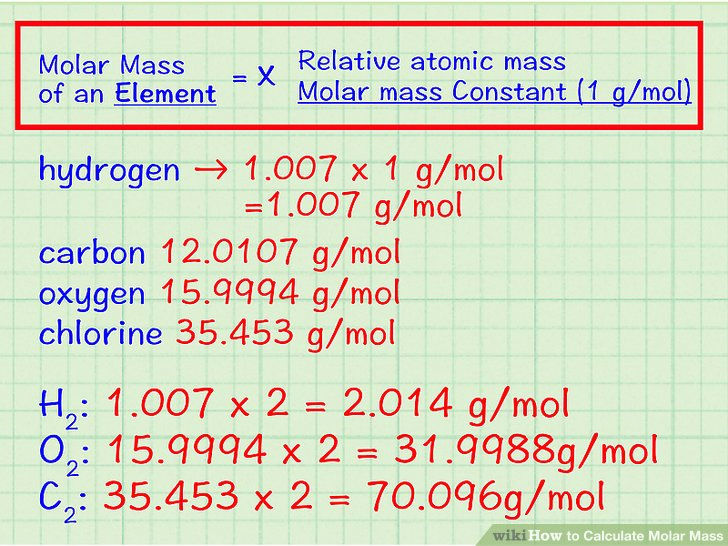
Relationship between the number of moles and the mass of a substance

Example
What is the mass 0.1 mol of magnesium? [Relative atomic mass: Mg=24]
Molar mass of Mg = 24 g mol-1
Mass of Mg = 0.1 mol × 24 g mol-1
= 2.4g


Comments